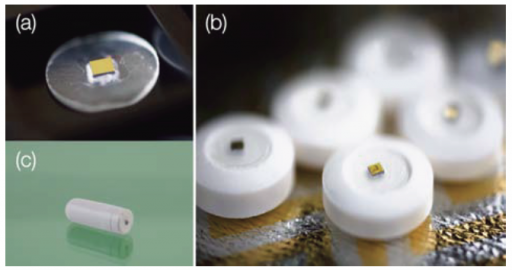
Proteus Digital Health, Inc. announced Monday that the U.S. Food and Drug Administration (FDA) has cleared its ingestible sensor for marketing as a medical device.
The ingestible sensor (formally referred to as the Ingestion Event Marker or IEM) is part of the Proteus digital health feedback system, an integrated, end-to-end personal health management system designed to help improve patients’ health habits and connections to caregivers.
“The FDA validation represents a major milestone in digital medicine,” said Dr. Eric Topol, professor of genomics at The Scripps Research Institute and author of The Creative Destruction of Medicine: How the Digital Revolution Will Create Better Healthcare. Directly digitizing pills, for the first time, in conjunction with our wireless infrastructure, may prove to be the new standard for influencing medication adherence and significantly aid chronic disease management,”
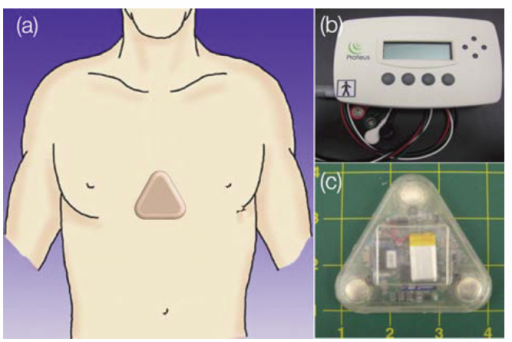
The Proteus ingestible sensor can be integrated into an inert pill or other ingested products, such as pharmaceuticals. Once the ingestible sensor reaches the stomach, it is powered by contact with stomach fluid and communicates a unique signal that determines identity and timing of ingestion.
This information is transferred through the user’s body tissue to a patch worn on the skin that detects the signal and marks the precise time an ingestible sensor has been taken. Additional physiologic and behavioral metrics collected by the patch include heart rate, body position and activity.
The patch relays information to a mobile phone application. With the patient’s consent, the information is accessible by caregivers and clinicians, helping individuals to develop and sustain healthy habits, families to make better health choices, and clinicians to provide more effective, data-driven care.
Via: "Kurzweil AI"
Intrusting
ReplyDeleteinstalab.pro